United States Food and Drug Administration, Federal Research Center
Silver Spring, Maryland
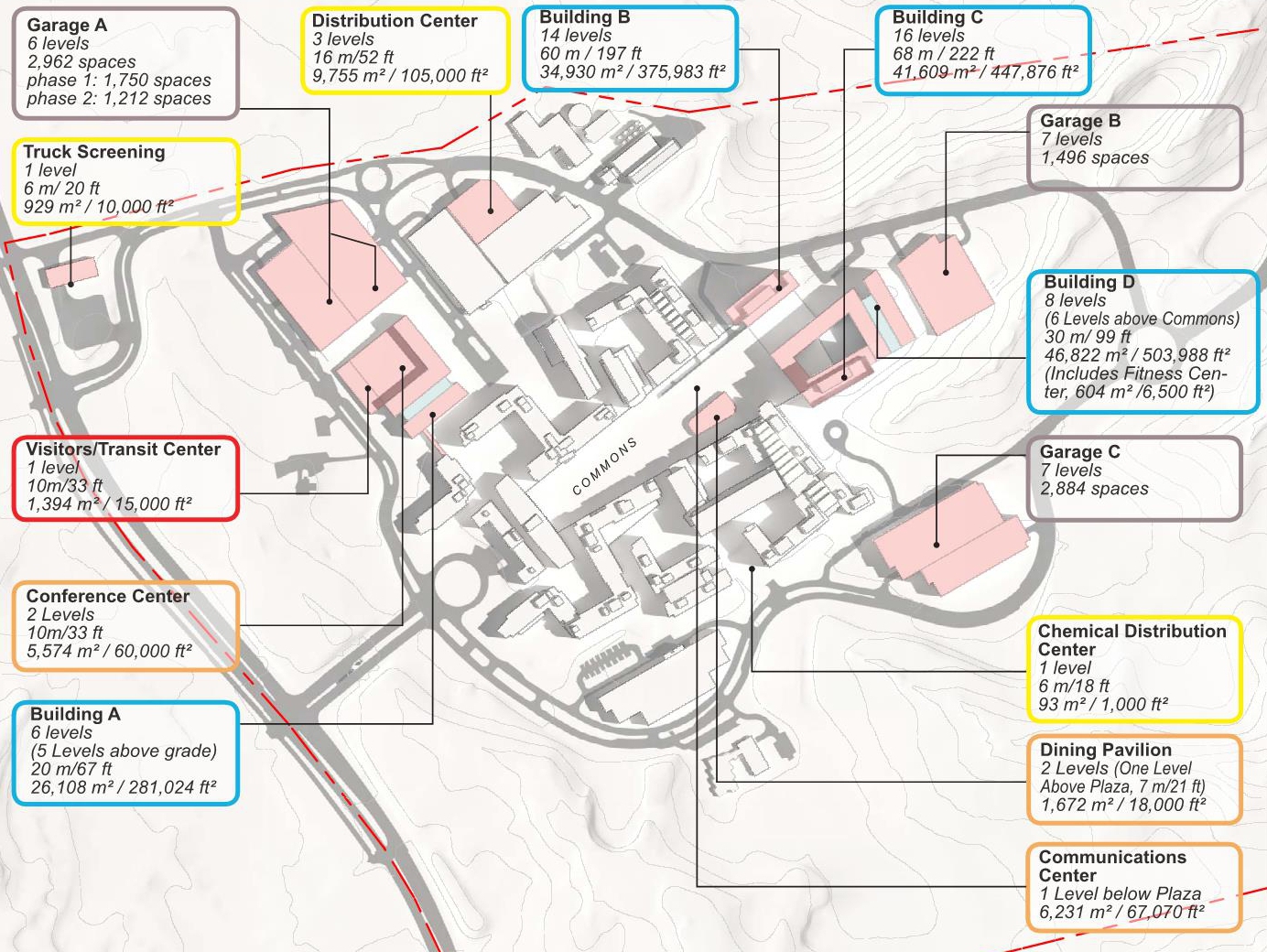
The new Master Plan will provide a guide for development to accommodate a total of 18,000 FDA employees and support staff at the 130-acre FDA Headquarters at the Federal Research Center (FRC) in Silver Spring, Maryland. The plan will steer the planning, design, and construction of new buildings; improvements to roadways, utilities, and other infrastructure; and the protection of natural areas. Preceding the Master Plan, a Land Use Feasibility Study was prepared that studied multiple development strategies within the FRC and a Draft Master Plan that developed three Master Plan Alternatives.
The purpose of the plan is to support consolidation of FDA employees and projected growth at the headquarters campus. The implementation of the Master Plan includes development of an additional 1,550,000 GSF of office space and 280,000 to 350,000 GSF of special use space to support FDA’s mission. Anticipating the implementation of bus-rapid-transit, parking would be provided at a ratio of 1 space for every 1.8 employees for a total of 10,000 parking spaces for FDA employees and campus support staff; Visitor parking would be increased from 1,000 to 1,615 parking spaces The expanded FDA Headquarters will be compatible with the architectural character and setting of the historic Naval Ordnance Laboratory through the continuation of the massing and material strategy established under previous Master Plans.
GBR was prime contractor and provided project management services for the feasibility study and Master Plan. Key consultants on the GBR team included CallisonRTKL (DC Office); Stantec (Laurel, Maryland Office) and Quinn Evans Architects (DC Office).